|
Color and paramagnetism – eumelanins
The most familiar characteristic of eumelanins is their black color and it has been well accepted that this is due to the extended conjugation of the electronic network throughout the biopolymer.
This argument has been used to explain the paramagnetism, blackness, and intractability of the material. In our studies we have begun to reexamine the nature of the mobility of the electrons within eumelanin polymers with specific attention paid to their possible origins.
From the original studies by Nicolaus (1) and Swan (2) came the proposal that eumelanin is a highly crosslinked heteropolymer of 5,6-dihydroxyindole, DHI, 5,6-dihydroxyindole-2-carboxylic acid, DHICA, and DOPA covalently bound in an irregular fashion. However, recent studies by Prota (3) and by Ito (4) have shown that both DHI and DHICA are generated from DOPA in substantial quantities. In the presence of metal-ions both compounds further react to give covalently bound oligomers. It is unreasonable to assume then that the formation of eumelanins from these precursor materials should be formed in a haphazart fashion, especially under biological conditions. Jimbow's SEM study on melanosome formation shows clearly the highly ordered structure of the eumelanin melanosome (5). As melanosome buildup is complete, the spaces fill between the initially formed strands making the total structure appear to have a highly compacted form. The harsh chemical treatments typically utilized in the removal of natural eumelanins from their native surroundings could severely alter the original chemical and physical nature of the melanosome such that subsequent analyses yield imprecise information.
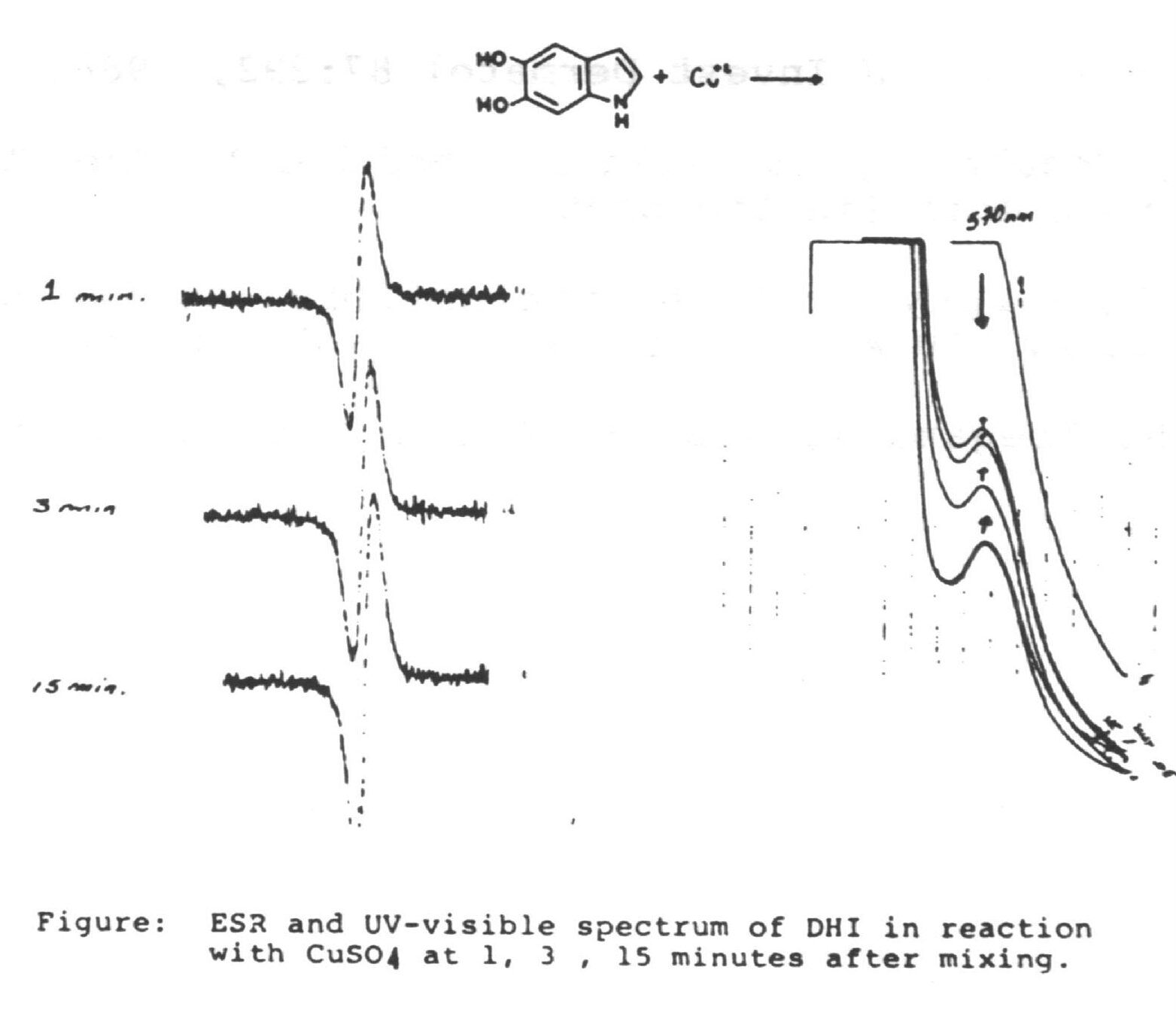
We have found the oxidation of DHI gives a highly structured material that consists of oligomers with a molecular weight of about 3,000 Daltons (6). This oligomer has approximately 1.5 water molecules bound to each unit of DHI. ESCA studies revealed that 20~25% of the DHI unites are in an oxidized state similar to that of adrenochrome (7). X-Ray diffraction studies material further shows repeat spacings at 15 and 3.4 A. which leads to a component cell length of 5 DHI molecules attached linearly across the benzene portion. Carbon 13 NMR studies using specifically enriched (13C2 and 13C3). DHI further indicated that there is no change in the substitution at the pyrrole portion of the compounds (8). This suggests a structure for the oligomer of a two stranded helix of 5 DHI units in various stages of oxidati aligned in either a head to head or head to tail configuration held via hydrogen bonding from the water molecules. This structure places the hydrophilic sections of the molecules exposed to the surrounding environment. Intersetingly we also have found that as the melanochrome forms from DHI there is an EPR signal quite similar to that of the intact eumelanin (Figure). Moreover these initial eumelanin oligomers have a paramagnetic characteristic of 1 spin per 6~ 8 units. With a carbonyl content of ca. 20% for these oligomers this value is much lower than what would be expected for a semi-quinone free radical. In solution the oligomers are dark brown, but upon acidification they precipitate out as black particles. One possibility for this color change is that in the soluble form the oligomers both absorb light and are large enough to scatter it. Upon precipitation they aggregate to larger particles which further scatter light and appear black. If the percieved color of the eumelanins were due solely to light absorption, the solution and solids would appear more similar. Additionally this would require more of an extended conjugation of the -electron system of the DHI units than is possible for the molecular dimensions we find for this material.
But the low value for the spin density certainly argues against solely the involvement of semi-quinone free radicals because they would not be delocalized throughout the oligomer. Rather the paramagnetism may be due to the establishment of charge transfer complexes across the helix between the oxidized and standard state of the DHIs. Studies by Kuroda et al (8) for merocyanine dye films show the presence of a simple free radical with a g-value close to that of a free electron. They argue that this is due to charge transfer through the stacked dye molecules. A similar phenomenon may be operative in DHI eumelanin. We are therefore continuing to further unravel the nature of the paramagnetism of eumelanins with specific attention to the possibility that the signals originate from an intrinsic conduction network inherent in the polymeric chain.
References
1. Nicolaus RA : Melanins. In : "The Chemistry of Natural Products", E.
Lederer (ed], Hermann Publishers, Paris (1968).
2. Swan GA : Structure, chemistry and biosynthesis of the melanins. Fortsch Chem Org
Naturst 31:521-582, 1974.
3. Palumbo A, Nardi G, d'Ischia M, Misuraca G, Prota G : Gen Pharm 14:253-257, 1983.
4. Ito S : Biochem Biophys Acta 883:155-161, 1986.
5. Jimbow K et al : Structure and function of melanin. Vol. 1; 15-23, 1983.
6. Schultz T et al : J Invest Dermatol 87:292, 1986.
7. Clark M, Schultz T, Gardella J, Chedekel M, Rao KV, Salvati L: Biochem Biophys Acta (in
the press)
8. Schultz T, Murphy B : 13th International Conference on Heterocyclic Chemistry, IUPAC,
August 10-14, 19BS, Guelph, Canada.
9. Kuroda S, Ibegami K, Sugi M, Iizima S : Solid State Comm 493-497, 1986.