Pulsed irradiation studies of unstable
intermediates
of melanin pigment formation
The fast reaction techniques of flash photolysis and pulse
radiolysis are known mainly for their enormous contributions to knowledge of free radical
and excited state chemistry, and, in particular, the involvement of such species in the
chemistry of biology and medicine (1). There is however a whole "spectrum" of
unstable intermediates including, for example, orthoquinones, quinone-imines and
quinone methides which are neither free radicals nor excited states, yet are too
short-lived to be studied by normal methods, but are amenable to study by these pulsed
radiation techniques. A variety of such transient intermediates are thought to be involved
in the complex processes of melanin formation, and this article attempts to call attention
to the largely unexplored potential of pulsed radiation techniques to elucidate these
processes.
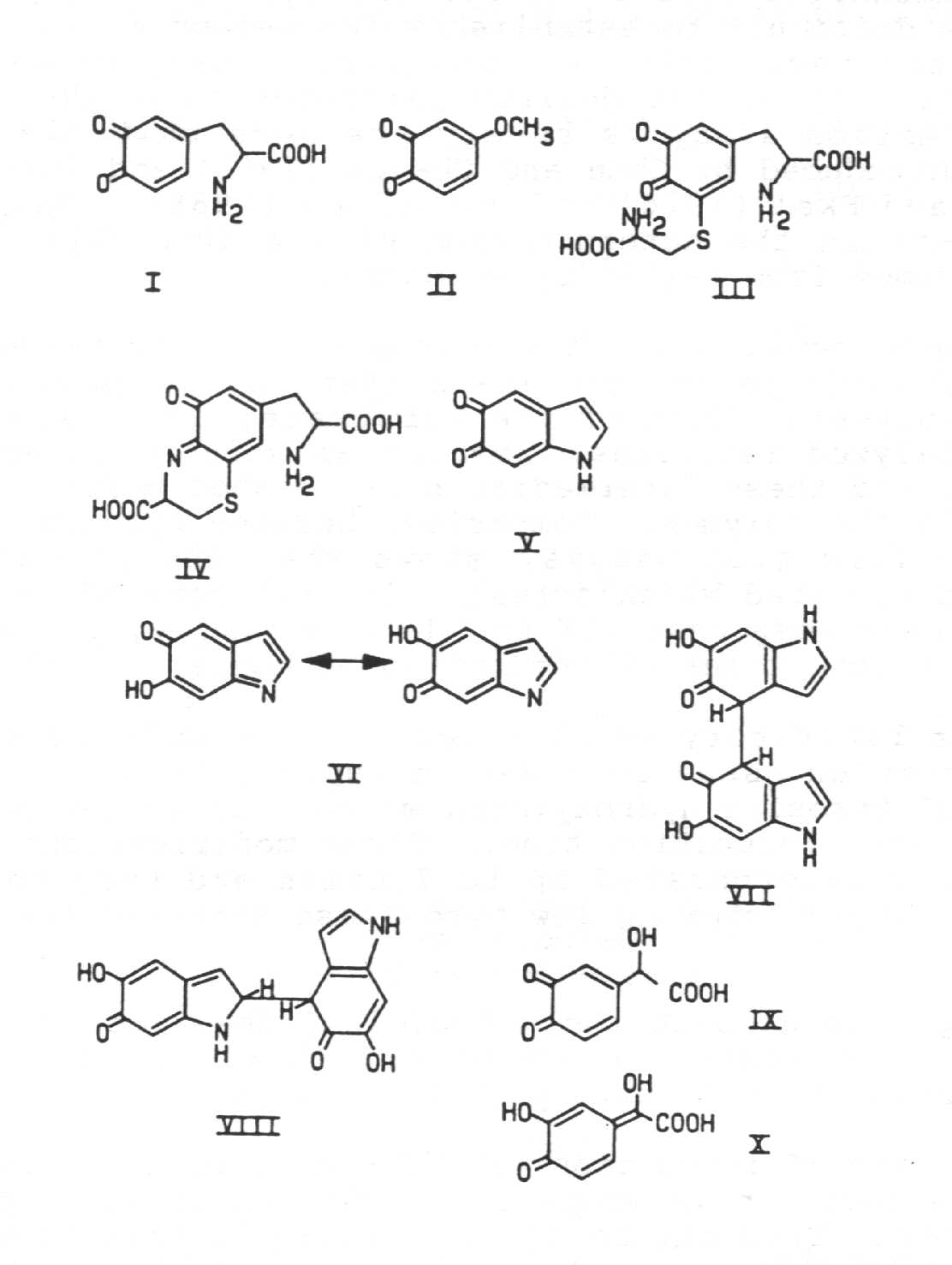
A common mode of decay of semiquinones is disproportion to give quinones and
dihydroxybenzenes. Several unstable orthoquinones, for example, dopaquinone (I)
(2-4) and the false melanin precursor anisyl-3,4-quinone (II) (5), can be prepared almost
instantaneously by one-electron oxidation of the corresponding dihydroxybenzenes with
sub-microsecond pulses of radiation. For dopaquinone, under the conditions chosen (3),
formation of dopaquinone from stable dopa was complete within ~5 ms of the
pulse, and its decay, eventually to form dopachrome, followed over tens of ms to seconds,
depending upon the pH. For anisyl-3,4-quinone, formation of the orthoquinone was
complete within 20 ms of pulse irradiation (Aqueous solutions of the latter cannot be made
by simple dissolution of solid quinone without significant polymerisation occurring during
the process of dissolution). In the formation of both orthoquinones, one-electron
oxidation can be per formed either photochemically, via photoionization and/or photo
radical homolysis, or radiation chemically, for example, via N3 oxidation, the
corresponding semiquinone being formed initially which disproportionates to form the orthoquinone
(and reform the parent dihydroxybenzene).
The corresponding orthoquinone (III) of 5-S-cysteinyldopa, which can only be
similarly formed radiation chemically (3,4), decays by a different and competing
cyclisation path to that pertaining to dopaquinone, the cysteinyl side chain amino
substituent attacking the carbonyl of the phenyl ring with loss of water to produce an
unstable guinone-imine (IV), postulated by Prota et al (6). This quinone-imine then
rearranges by a hydrogen shift to a more stable benzothiazine isomer.
Another important quinone which has long been postulated to be crucial to melanin pigment
formation is 5,6 indolequinone (V).
One-electron oxidation of 5,6 dihydroxyindole is thought to lead to the corresponding
oxygen-centred semiquinone radical which probably disproportionates to 5,6 indolequinone
and reform 5,6 dihydroxyindole (7). The 5,6 indolequinone appears to be unstable, decaying
by 1rst order kinetics into a substance, or substances, possessing considerable absorption
around 540 nm where melanochrome has a maximum (8,9). One of these substances could be the
rearrangement product (VI), reminiscent of dopachrome but with added conjugation, which
may be a seed of the polymerisation which ultimately leads to melanin. Positive
assignments of the above unstable species are difficult however to establish
unequivocally, and it could be that the semiquinone from 5,6 dihydroxyindole, in part at
least, decays by demerisation, the dimers initially formed, possibly (VII) and (VIII) for
instance, being unstable and rearranging by unimolecular processes into more stable dimers
of the type that have been isolated by Corradini et al (10) and d'Ischia and Prota (11).
Decarboxylations are also involved in melanin pigment formation and pulsed radiation
techniques may help in elucidating such processes. For instance, tyrosinase catalyses the
oxidative decarboxylation of 3,4-dihydroxymandelic acid leading to
3,4-dihydroxybenzaldehyde (12) although 4-hydroxymandelic acid and
3-methoxy-4-hydroxymandelic acid are inert. One-electron oxidation of
3,4-dihydroxymandelic acid via pulse radiolysis (13) led to the corresponding semiquinone
which decayed by disproportionation into a species which subsequently decayed by 1rst
order kinetics (k~2 s-1 ) into a stable product with an absorption
spectrum matching that of 3,4-dihydroxybenzaldehyde. Since one-electron oxidation of
4-hydroxymandelic acid did not appear to lead to 4-hydroxybenzaldehyde, it is suggested
that the intermediate resulting from the second order decay of semioxidised
3,4-dihydroxymandelic acid is the orthoquinone (IX) rather than the corresponding
quinone methide (X) previously postulated (12), the equivalent of which should also have
been formed from 4-hydroxymandelic acid.
It is hoped that the above gives some idea of the scope of pulsed radiation techniques for
studies of unstable melanin precursors. Since the equipment in particular the sources of
pulsed ionizing radiation for carrying out such experiments are expensive and
comparatively rare, if any reader wishes to consider jointly pursing other ideas for
investigating unstable intermediates related to melanogenesis please get in touch.
Acknowledgements
I am grateful to J.M. Bruce, J.N. Chacon, M.R. Chedekel, C., Lambert, P.A. Riley, T.
Sarna, A. Thompson and T.G. Truscott for collaboration, G. Prota for comments, and the
Cancer Research Campaign and Medical Research Council for support.
References
(1) Bensasson RV, Land EJ, Truscott TG. Flash photolysis
and pulse radiolysis : contribution to the chemistry of biology and medicine. Pergamon
Press 1983.
(2) Chedekel MR, Land EJ, Thompson A, Truscott TG. Early steps in the free radical
polymerisation of 3,4-dihydroxyphenylalanine (Dopa) into melanin. J Chem Soc Chem Comm
1170-1172, 1984.
(3) Thompson A, Land EJ, Chedekel MR, Subbarao KV, Truscott TG. A pulse radiolysis
investigation of the oxidation of the melanin precursors 3,4-dihydroxyphenylalanine (dopa
) and the cysteinyldopas. Biochim Biophys Acta 843:49-57, 1985.
(4) Land EJ, Thompson A, Truscott TG, Subbarao KV, Chedekel MR. Photochemistry of melanin
precursors : dopa, 5-S-cysteinyldopa and 2,5-S,S'-dicysteinyldopa. Photochem Photobiol
44:697-702, 1986.
(5) Cooksey CJ, Land EJ, Riley PA, Sarna T, Truscott TG. On the interaction of
anisyl-3,4-semiquinone with oxygen. Free Rad Res Comm 4:131-138, 1987.
(6) Prota G, Crescenzi S, Misuraca G, Nicolaus RA. New intermediates in phaeomelanogenesis
in vitro. Experientia 26:1058-1059, 1970.
(7) Lambert C, Chacon JN, Chedekel MR, Land EJ, Riley PA, Thompson A, Truscott TG. To be
published
(8) Napolitana A, Corradini MG, Prota G. A reinvestigation of the structure of
melanochrome. Tetrahedron Lett 26:2805-2808, 1985.
(9) Vachtenheim J, Duchon J, Matous B. A spectrophotometric assay for mammalian tyrosinase
utilizing the formation of melanochrome from L-dopa. Anal Biochem 146:405-410, 1985.
(10) Corradini MG, Napolitano A, Prota G. A biosynthetic approach to the structure of
eumelanins. The isolation of oligomers from 5,6-dihydroxy-1-methylindole. Tetrahedron
42:2083-2088, 1986.
(11) d'Ischia M, Prota G. Photooxidation of 5,6-dihydroxy-1-methyl-indole. Tetrahedron
43:431-434, 1987.
(12) Sugumaran M. Tyrosinase catalyses an unusual oxidative decarboxylation of
3,4-dihydroxymandelate. Biochem 25:4489-4492, 1986.
(13) Bouheroum M, Bruce JM, Land EJ To be published.

E.J. LAND
Paterson Institute for Cancer Center
Christie Hospital & Holt Radium Institute
Manchester M20 9BX
England, U.K.
Phone : 061 445 8123 ext 215